PT-141, also known as bremelanotide, is a unique peptide that may help improve symptoms of sexual dysfunction and related conditions by targeting the melanocortin receptors in the human body.
PT-141 has been studied for its potential to improve sexual function in both men and women and it is currently approved by the US Food and Drug Administration (FDA) for therapy in females.
More specifically, the peptide has been FDA-approved since 2019 under the brand name Vyleesi for managing acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women.
Research has also suggested that PT-141 may have a positive impact on erectile function, sexual desire, and overall sexual satisfaction in men. However, further research is needed to establish its efficacy and safety in males.
So can we say that PT-141 is the best peptide for sexual health? Is it equally safe and effective in men and women alike?
Keep reading to discover the latest information on how PT-141 works, what are its benefits for males and females, and what are risks to be aware of.
What is PT-141?
PT-141 is a peptide medication that is legally available in the US under the brand name Vyleesi. Vyleesi is a trade name of Palatin Technologies, Inc. – a US biopharmaceutical company that is the original manufacturer and patent holder of the peptide.
It is a prescription-only drug that is indicated for managing HSDD in premenopausal women, but it can also be prescribed off-label in men and women with other sexual problems.
PT-141 (Vyleesi) can be obtained legally only from a licensed pharmacy, after presenting a prescription from a healthcare professional.
In addition, the peptide can be purchased online under the pretense that it will be used as a reference material for laboratory research. In reality, the buyers often take it for personal use, despite the fact that administering such forms of PT-141 to humans is illegal.
In fact, the only legal option for human use is Vyleesi, which is the brand name for the PT-141 that is approved and regulated by the FDA. There is no generic version, and all other versions of PT-141 are not under any regulation for quality or safety.
Thus, they are not only illegal but also potentially dangerous for human use. Many of those peptides are manufactured overseas or in underground laboratories without proper quality control or adherence to regulations. Such peptides may be diluted, contaminated, or simply counterfeit.

PT-141
Improve erections and libido in men
Increase sex drive in women
Reduce appetite
How does PT-141 work?
PT-141 is a fully-synthetic, cyclic heptapeptide. It is a carboxylated metabolite of another MCR agonist called Melanotan II that mimics the function of the α-melanocyte stimulating hormone (α-MSH). α-MSH occurs naturally in the human body and works by activating the melanocortin receptors (MCRs).
There are 5 melanocortin receptors ranging from MC1R to MC5R. Similar to α-MSH, PT-141 appears to activate all MCRs except MC2R, which is found in the adrenal glands and is specific for the adrenocorticotropic hormone (ACTH).
However, α-MSH has a stronger affinity towards the MC1R in the skin that plays a role in regulating melanin production, while PT-141 is biased toward the MC3R and MC4R.
MC4R is found in the brain and plays a crucial role in regulating various physiological processes, including sexual function, energy homeostasis, and appetite regulation.
By binding to MC4R, PT-141 stimulates pathways in the brain that are involved in sexual arousal, desire, and motivation. Activation of MC4R by PT-141 can lead to increased sexual desire and potentially improve sexual function, although the exact mechanisms via which it achieves these effects are still largely unknown.
Studies suggest that it takes about 45 minutes for PT-141 to start working.
The peptide exerts its effects rapidly after administration, and the recommendations are to take it within 45 mins prior to sexual activity. Furthermore, the increase in sexual arousal usually lasts for 2-4 hours, rarely longer.
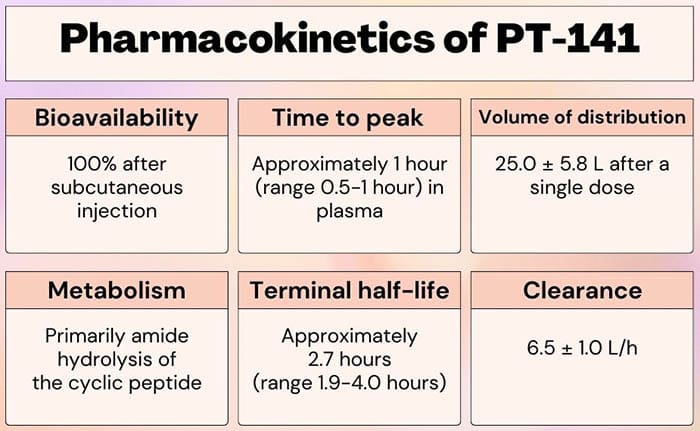
This coincides with the half-life of the peptide, which has been reported to be about 2.5 hours after subcutaneous injection. Considering the fact that it takes about 4-5 half-lives for the body to eliminate most substances completely, then the peptide should be gone from a person’s system within 12-13 hours on average.
Due to its rapid onset, the peptide is recommended for use on demand (when needed). However, it should not be used more often than once per day and 8 times per month to minimize the risk of side effects.
Apart from subcutaneous injections, PT-141 has also been investigated for its potential use as a nasal spray. Such a formulation would allow for similar effectiveness coupled with easier use and rapid absorption.
However, the official development of intranasal PT-141 was canceled in 2008 due to concerns of adverse effects such as hypertension raised by phase-2 trials.
PT-141 benefits
The proven and potential benefits of PT-141 include:
- increased sexual desire
- higher vaginal blood flow in women
- improved erectile function in men
- reduced appetite
The main benefit of PT-141 is its ability to improve sexual desire and libido in premenopausal women suffering from HSDD. In addition, the peptide may also increase libido in men and women without HSDD.
The exact mechanism by which bremelanotide improves sexual desire and arousal in premenopausal women with HSDD is not fully known. It is believed that bremelanotide, as a melanocortin receptor agonist, may increase the release of dopamine in the brain, which alters key pathways involved in sexual response.
As a result, PT-141 can potentially increase sexual desire, enhance sexual arousal, and improve sexual satisfaction. It is important to note that bremelanotide does not directly address other factors that may contribute to sexual dysfunction, such as physical causes or relationship issues.
Studies have also reported that PT-141 may stimulate vaginal blood flow in healthy women as well as increase libido and arousal in menopausal women.
It’s unknown if the peptide also increases blood flow in the genital area of men, but studies report that it has a potent erectogenic effect and may be a candidate for therapy in males with erectile dysfunction (ED).
The mechanism of improving erections in men is likely related to its effect on libido and arousal. There is no data to suggest that PT-141 may influence levels of testosterone.
PT-141 may also help suppress appetite levels by activating the MC4R. As a result, it may help you lower your energy intake and facilitate weight loss. As of 2023, there are active phase-1 clinical trials that investigate the potential effectiveness of this peptide for weight loss.
One of the trials was completed by 27 obese women, which reduced their daily energy intake by 400 kcals and lost about 3.7 lbs of body weight for 16 days, compared to 2 lbs in the placebo group.
PT-141 effects for men
PT-141 is currently not approved for use in men, although it can be prescribed off-label. Currently, there is some research suggesting that the peptide can improve sexual function and erections in males.
One of the few trials in men investigated the effect of subcutaneous PT-141 in both healthy male subjects and patients with ED who did not respond adequately to Viagra.
Significant erectile responses were observed in both healthy subjects and patients with erectile problems when treated with PT-141 in combination with visual sexual stimulation.
These effects were observed after injecting 1 mg of PT-141 in the healthy group and 4-6 mg in those suffering from ED. Also, learn how HGH therapy affects penis size and whether it can make the penis bigger in adulthood.
PT-141 has also been studied in the form of intranasal spray for men and reported positive effects in 33.5% of patients in the bremelanotide group compared with 8.5% of the patients in the placebo group. However, there also were concerns regarding the safety of such intranasal formulations.
PT-141 effects for women
Both intranasal and subcutaneous PT-141 formulations have been tested successfully for the management of female sexual dysfunction. Yet, nasal sprays are significantly less researched and currently not approved for human use.
Numerous studies, including phase-2b and phase-3 trials, have tested the effectiveness of PT-141 on women suffering from HSDD and female sexual arousal disorder (FSAD). They have investigated the effects of various dosages, including 0.75 mg, 1.25 mg, or 1.75 mg of PT-141, compared to placebo.
Results have shown that bremelanotide has the greatest efficacy profile in doses of 1.25 mg and 1.75 mg. The efficacy included statistically significant improvements in the rate of sexually satisfying events and increased scores of sexual desire, assessed by the Female Sexual Function Index (FSFI).
Ultimately, injecting 1.75 mg was selected as the most effective dosage. This dosage was also used in the phase-3 trials which led to the FDA approval of 1.75 mg bremelanotide injections for premenopausal women with HSDD.
PT-141 side effects
The safety of the peptide PT-141 has been studied extensively in women, particularly in a phase 3 trial involving 1,247 participants.
The study aimed to investigate the safety profile of PT-141 and compare it to a placebo. Results revealed that the treatment group had a higher percentage of adverse side effects compared to the placebo group (76.6% vs. 58.2%).
Among the most commonly reported adverse events associated with PT-141 were nausea (40% vs. 1.3%), flushing (20.3% vs. 0.3%), and headache (11.3% vs. 1.9%) when compared to the placebo group.
It’s important to note that despite these side effects, only a small proportion (8.1%) of the PT-141 group discontinued the medication. There was a modest, transitory increase in blood pressure after use.
Darker skin and hyperpigmentation due to the activation of the MC1R in the skin are also potential side effects of PT-141.
Overall, the researchers report that the majority of side effects related to PT-141 were transitory, such as reduced tolerance towards the drug and were mild to moderate in severity.
Other studies report that focal hyperpigmentation was rare when bremelanotide was administered according to the recommendations, but it was still observed in over one-third of subjects following up to 16 consecutive daily doses.
How to use PT-141
Currently, PT-141 (Vyleesi) is approved only for subcutaneous use as a prefilled single-dose auto-injector pen that delivers 1.75 mg of the peptide in a 0.3 mL solution.
The active ingredient is bremelanotide acetate, while the inactive ones are 2.5% glycerin, sterile water for injection, and hydrochloric acid or sodium hydroxide added to adjust the pH.
Since the pen is prefilled, PT-141 does not require mixing or reconstitution
The recommended injection sites for subcutaneous injection include the abdomen (except for a two-inch area around the navel), the front of the thigh, or the upper arm.
Prior to each dose, patients must clean the injection site with rubbing alcohol and change the injection site for each dose to minimize skin injury.
The peptide should not be injected into skin that is irritated, sore, bruised, red, or scarred.
To minimize the risk of side effects such as hypertension and hyperpigmentation, PT-141 should not be injected more frequently than once within a 24-hour period and no more than eight times per month.
In addition, the PT-141 can be purchased online as a lyophilized powder that requires manual reconstitution with a diluent such as bacteriostatic water.
However, such formulations are not under any regulation, as they are sold under the pretense of being reference materials for research. These products are illegal and potentially dangerous for human use.
How to store PT-141
The PT-141 (Vyleesi) pens can be stored at under 77°F (25°C) before use and do not require refrigeration. Their shelf life, even when not refrigerated, is about 12 months.
The pens should not be frozen or exposed to extreme temperatures and direct sunlight, as that can damage the peptide. Since each pen is single-use, it can be discarded right after an injection.
Peptides that are manually reconstituted with bacteriostatic water (0.9% benzyl alcohol) must be refrigerated at 36°F to 46°F (2°C to 8°C) and remain viable for up to 28 days.



 Request Appointment
Request Appointment